Training the next generationof musculoskeletal interface scientists
We aim to unravel MSK interface structure–function relationships from the macro- to molecular scale, addressing fundamental questions in:
- Bone–tendon and muscle–tendon interfaces
- Bone quality and mineral–collagen interactions
- Osteocyte lacunar-canalicular networks & mechanobiology
- Age-, sex- and gender-specific differences in MSK health
- Regeneration and remodeling in zebrafish and mammalian models
- AI-enhanced image analysis
- Computational modeling of interfaces
Our projects address these by integrating different approaches, ranging from synchrotron imaging, ultra-high-resolution electron microscopy (TEM, SBF-SEM, FIB-SEM), nano- and micro-CT, SAXS/WAXS, Raman spectroscopy, optical coherence tomography, advanced biomechanics, Finite-Element-Methods & Finite Cell Methods to AI-driven analytics into unified, correlative workflows.
Main supervisor: Isabel Molwitz (UKE)
Co-Supervisors: Tobias Knopp (TUHH/UKE) & Alexander Schlaefer (TUHH)
Research background and question
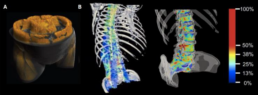
Muscle quality has recently gained interest as a potentially superior, predictive imaging biomarker for, e.g., survival, compared to muscle mass [3-5]. CT allows to quantify bone and muscle mass. Muscle quality used to be assessed indirectly by CT density reflecting myosteatosis. CT density, however, is affected by contrast agents. Spectral CT scanners now enable non-invasive quantification of bone and muscle fat, and BMD unbiased by contrast agent [1,2,7]. Pioneering work on the predictive value of spectral CT muscle fat quantification, demonstrated superior impact of quantified muscle fat over muscle density or muscle mass, as conventional sarcopenia criterion, for mortality and morbidity in critically ill patients [6]. This project will extend the approach of utilizing quantification parameters readily available from clinical routine spectral CT scans with a first investigation of the predictive value of bone marrow fat and the combination of bone and muscle quality parameters for fracture risk, adverse outcome, and survival. Also, it will serve to implement enhanced AI-based image analysis to increase predictive value and clinical applicability further.
Specific aims
- Determine the predictive value of bone marrow fat quantified using spectral-CT techniques with enhanced AI-based image analysis for fracture risk, adverse outcome, and survival.
- Evaluate the complex interaction of bone, muscle, and fat contents for a more precise prediction of fracture risks, adverse outcomes, and reduced survival.
Work program and methods
In this project, the student will perform spectral-CT material quantification for soft tissue, fat, and bone mineral in retrospectively available clinical image datasets. The focus will be on older patients (>60 years; sample size testing: n=762) who received CT scans including the column vertebrae, e.g., exams of the aorta. Data on adverse outcomes such as hospitalizations, surgery, and survival will be derived from electronic patient files. Fractures will be detected on initial CT scans and if available follow-up examinations. Deep-learning AI-based tools for 3D segmentation and radiomics for pattern analysis for the muscle will be available from an ongoing DFG-funded project of the supervisor. With the co-supervisors the student will learn to develop similar algorithms for the vertebral bone. The dataset will then be analyzed for 3D BMD, muscle mass, and bone and muscle fat content, including radiomics. This will allow for the evaluation of the predictive value of each component, the selection of leading components, and the creation of a superior prediction model for fracture risk and adverse outcomes.
P2 Exploring molecular pathways of tendon-to-bone healing in normal and osteoporotic bone remodeling
Main Supervisor: Johannes Keller (UKE)
Co-Supervisors: Kartharina Jähn-Rickert (UKE) & Sara Checa (TUHH)
Research background and question
Rotator cuff (RC) injuries are among the most common shoulder injuries, especially in older patients with osteoporosis [1], where bone resorption exceeds bone formation. Although there have been significant advances in surgical techniques, there is still a high incidence of RC re-rupture following reconstruction [2,3]. A common site of RC re-rupture is the enthesis, where the tendon is attaching to bone. Numerous studies have been conducted to improve tendon integration, but these have primarily focused on the pathophysiological processes within the affected tendon itself [4]. In contrast, the influence of bone status and local remodeling processes at the bony insertion site on healing outcomes is poorly understood. This is surprising, because tendon is a bradytrophic tissue and adequate healing of tendon into bone is thought to depend primarily on bone processes, including dynamic changes in bone formation by osteoblasts and bone resorption by osteoclasts.
Specific aims
- Multiscale characterization of the impact of osteoporotic bone remodeling on tendon-to-bone and tendon-to-tendon healing (experimental).
- Explore the structure and cellular composition of the tendon-bone interface in patients with RC rerupture (clinical).
Work program and methods
The experimental section relies on the murine Achilles tenodesis model which has significant advantages over other tenodesis (e.g., EC reconstruction) models, including high reproducibility, practicality and the provision of clinically relevant outcome measures. Tendon-bone healing will be characterized in 12–14-week-old WT mice with/without OVX at defined time points. μCT-scanning (osseous changes), histology (structural and cellular histomorphometry), spatiotemporal ultrastructural analysis (mineralization in tendon-to-bone transition, organization of linking collagen fibers at the interface) and biomechanical evaluation of failure force of the evolving tendon-bone interface, will be performed. A cohort in which the Achilles tendon is dissected and reconstructed in the middle (established) will serve as control to differentiate osseous regenerative processes from tendon-to-tendon healing. The clinical section of this project aims to confirm the findings obtained in the mouse model in surgical specimens obtained during revision surgery for reruptured RC tenodesis (supraspinatus). During RC revision surgery, the bone at the previous supraspinatus tendon insertion site is refreshed to cause bleeding, which facilitates tendon-to bone healing. The removed bone including the attached remnants of the ruptured tendon will be used for subsequent multimodal analysis as described above.

Main Supervisor: Benjamin Ondruschka (UKE)
Co-Supervisors: Alexander Schlaefer (TUHH) & Sara Checa (TUHH)
Research background and question
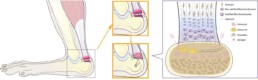
Tendon-bone interfaces become increasingly vulnerable to age-related degeneration. Older adults are particularly at risk for tendon disorders and enthesis-related pathologies, which contribute to reduced mobility, chronic pain, and heightened fracture susceptibility. Conditions affecting tendon integrity often lead to functional impairment and the need for surgical intervention. In patients with osteoporosis, tendon detachment and enthesis failure are further aggravated by bone fragility and altered remodeling, increasing the risk of severe injuries such as humeral or femoral fractures following falls. In addition to the hard-soft tissue enthesis interface, the soft-soft tissue myotendinous junction (MTJ) interface is a location where many injuries occur. Yet, the knowledge about its structure and adaptive capacity to training and inactivity is sparse. By investigating how aging and physical activity impacts the integrity and mechanical functionality of tendon interfaces, this research aims to provide a deeper understanding of the mechanisms that contribute to tendon failure and age-related contributors to musculoskeletal fragility.
Specific aims
- Quantify age-related structural and cellular changes in human tendon entheses, cortical bone, and myotendinous junctions
- Determine biomechanical properties and failure behavior of aging tendon–bone and muscle–tendon interfaces using uniaxial tensile testing and digital image correlation
- Correlate tissue microstructure with mechanical loading, force transmission, and fracture susceptibility
Work program and methods
This project will investigate the tendon enthesis, cortical bone microstructure, as well as myotendinous junction to correlate structural alterations at tissue interfaces with biomechanical function and fracture susceptibility. The human cadaveric cohorts will first be categorized into different age groups and scanned using DEXA and CT to extract information on the skeletal status. Post-mortem bone-tendon-muscle samples will be collected through forensic medical investigations. Specimens will include the lesser trochanter of the femur and the attached psoas major, selected due to its critical role in locomotion, its exposure to varying mechanical demands, and its known adaptive responses to physical strain. Biomechanical uniaxial tensile testing will be conducted to assess tensile strength, stiffness, and load-bearing capacity of tendon-bone and muscle-tendon specimens according to standardized test protocols. Digital image correlation and stress-strain mapping will provide strain distribution data, allowing for a precise evaluation of mechanical failure points. Histological staining and immunohistochemistry will be performed to assess structural and cellular changes, e.g., osseous cells, chondrocyte and tenocyte activity, collagen organization, cellular composition, and extracellular matrix changes in the enthesis, bone, and MTJ.
Main Supervisor: Björn Busse (UKE)
Co-Supervisors: Imke Greving (Hereon) & Benjamin Ondruschka (UKE)
Research background and question
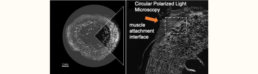
Mechanical loading is known to influence the nano- and microstructural properties of the enthesis and MTJ, but how these interfaces adapt to different physiological states remains unclear. Moreover, the ultrastructural characteristics of mineralized tendon-bone interfaces in humans remain poorly understood [1]. The spatial arrangement of mineralized and non-mineralized tissue, collagen fiber orientation, and the relationship between mineral composition and mechanical stability require further investigation. Structural adaptations in physically active individuals may exhibit reinforced mineralization patterns and collagen organization, while immobilized and aging individuals may show reduced mineralization and impaired tendon-bone integration and degenerative changes contributing to joint instability and fracture susceptibility. However, quantitative data on these adaptations, particularly in human tissue samples, remain limited.
Specific aims
- Characterize the 2D/3D ultrastructure of mineralized and non-mineralized tissues at the enthesis and myotendinous junction to identify structural markers linked to interface integrity.
- Investigate microarchitectural differences across distinct physiological states, comparing immobilized individuals, physically active individuals, and elderly populations to understand how mechanical loading and aging influence enthesis adaptation and degeneration.
Work program and methods
This project aims to characterize the ultrastructural features of the mineralized bone-tendon interface across different physiological states using high-resolution imaging across high-risk cohorts (immobilized individuals with prolonged unloading due to spinal cord injury, physically active individuals exposed to high mechanical loading, and elderly individuals experiencing age-related degenerative changes). Circular Polarized light microscopy (CPL), synchrotron micro- and nanoCT, quantitative backscattered electron imaging (qBEI), and focused ion beam scanning electron microscopy (FIB-SEM) will be used to analyze the 2D/3D-dimensional ultrastructural organization at tendon interfaces. A correlative imaging approach will be employed to characterize the ultrastructure and mineral composition of the enthesis. The same suite of imaging and microscopy methods will then be used to analyze the 2D/3D-dimensional ultrastructural organization of fibrous tissues and mineralization patterns at hard-soft and soft-soft interfaces of tendons. The datasets will be employed for the development of machine learning / computational models within the RTG.
Main Supervisor: Katharina Jähn-Rickert (UKE)
Co-Supervisors: Maike Frye (UKE) & Tobias Knopp (UKE/TUHH)
Research background and question
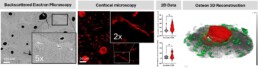
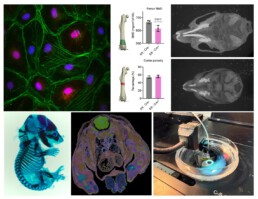
Osteocytes are highly secretory, mechanosensitive cells orchestrate various other cell types with their extensive dendritic network within the bone matrix [1]. Osteocytes directly interact with the vasculature in bone i.e., the intra-cortical vessels, to allow for communication and an exchange of secretory molecules. The structure-function relationship of the network-forming osteocytes implies that a vital network is required to orchestrate physiological reactions. The same is true for the vasculature network, consisting of both blood and lymph vessels [2]. Osteocyte network disruptions are complex and progressive and may include loss of dendritic and, or canalicular connections [3], loss of viable osteocytes, empty lacunae, up to micropetrosis of osteocyte lacunae, as seen in diabetic bone disease (see Figure) [4]. Similarly, blood perfusion and bone vasculature density are lowered with osteoporosis, osteoarthritis and other bone diseases, which are also characterized with an osteocyte network phenotype, suggesting that both 3D networks are intricately linked.
Specific aims
- Assess pathological changes and identify structure-function alterations in osteocyte and vascular networks using dual-imaging approaches and AI-driven image processing.
- Determine structural mechanisms and processes involved in osteocyte and vascular network replenishment under pathological conditions.
Work program and methods
This project will employ dual-imaging approaches and AI-driven image analysis to examine pathological changes in the osteocyte and vascular networks simultaneously. Using high-resolution imaging e.g. Micro-computed tomography, scanning electron microscopy, and scanning confocal laser microscopy, networks will be visualized. Thereby, the project will focus on establishing dual-imaging approaches both feasible and providing a matching high spatial resolution. Image processing will be a central part of the methodology and will aid the distinction of network characteristics. Quantification of 3D data sets is the final step towards data analysis.
Main Supervisor: Maike Frye (UKE)
Co-Supervisors: Imke Fiedler (UKE) & Björn Busse (UKE)
Research background and question
Endothelial cells (ECs) line the blood and lymphatic vasculature and act as central regulators of tissue homeostasis and organ function. Beyond serving as passive conduits, ECs provide paracrine (angiocrine) signals and sense mechanical and metabolic cues from their microenvironment [1]. Unlike other organs, the bone microenvironment is highly stiff, creating a unique environment for ECs [2,3,4]. While we have studied how matrix stiffness influences EC function in soft tissues [5,6], the influence of the bone microenvironment on bone EC behavior, as well as the reciprocal effects of vascular dysfunction and vascular stiffening on bone quality, remain to be investigated.
Specific aims
- Investigate the impact of bone matrix stiffness on endothelial cell behavior, focusing on mechanosensitive pathways in blood and lymphatic vessels.
- Determine how vascular dysfunction affects bone quality, exploring mechanisms that contribute to skeletal fragility and potential strategies for reversing these effects.
Work program and methods
This project entails ex vivo bone tissue analysis, spatial multiomics, advanced co-culture models, and in vivo disease modeling to examine the interaction between endothelial cells and the bone microenvironment.
The first phase will focus on bone and endothelial-proximal tissue stiffness mapping, using mouse and human bone samples to assess the mechanical properties of the vascular-bone interface. Co-culture models of ECs and bone cells will be developed within hydrogels engineered to mimic physiological and pathological bone stiffness, allowing for the controlled study of mechanosensitive endothelial responses. Next, the project will investigate the reciprocal effects of vascular dysfunction on bone quality using zebrafish and mouse models with altered bone stiffness and vascular pathologies. The zebrafish models will leverage newly developed genetic and dietary interventions (P9) to induce changes in bone stiffness and vascular integrity, providing a unique platform for live imaging of vascular remodeling and bone adaptation. Mouse models of vascular disease and endothelial dysfunction will further be used to assess long-term effects on bone health. This project will provide new insights into the role of ECs in bone health, vascular contributions to bone fragility, and potential therapeutic strategies for restoring skeletal-vascular integrity.

Images adapted from [3].
Main Supervisor: Alexander Schlaefer (TUHH)
Co-Supervisors: Sara Checa (TUHH) & Benjamin Ondruschka (UKE)
Research background and question
Elastographic imaging estimates the elastic tissue properties and can be realized with different clinical image modalities, including optical coherence tomography (OCT) and ultrasound (US). Shear wave elastographic imaging (SWEI) is an approach relating shear wave propagation to tissue elasticity, which allows for quantitative estimates. However, variance and deviations in the estimates typically increase for small fields of view [1] and at tissue interfaces, where wave propagation is complex due to reflection and refraction. Moreover, assumptions like isotropic tissue behavior and in-compressibility are often not fulfilled in real soft tissue [2]. Machine learning (ML) approaches help overcoming these issues [3,4]. However, high-resolution volumetric elastography of musculo-skeletal tissue at interfaces, in different load states, and for larger regions is still largely unexplored. Extending ML methods has the potential to improve analysis of soft-tissue interfaces, e.g., bone-tendon, tendon-muscle or tendon-tendon, and to compare soft tissue properties to obtain information on tissue health.
Specific aim
- Establish suitable imaging parameters and machine learning architectures to estimate elastic tissue properties at interfaces in 2D, 3D and 4D from raw signals and with high resolution.
- Consider shear wave propagation at tissue interfaces and study end-to-end deep learning for detection and compensation of interface related artifacts.
Work program and methods
The project will focus on extending work on Deep Learning (DL) methods for motion tracking [5] and elastography [6] to volumetric elastography of musculoskeletal tissue, see Figure above. Using fully configurable research US and OCT devices we will setup fast volumetric imaging with a small field of view. A robotic lab setup will be adapted to obtain large amounts of data from phantoms and ex-vivo tissue samples with interfaces. We will study recent DL approaches to estimate elasticity in small regions, to obtain high-resolution estimates of larger structures like tendons and muscle by motion tracking and volume stitching, and whether artifacts at interfaces can be mitigated by high-resolution and spatio-temporal DL models. The methods will be evaluated on musculoskeletal tissue interfaces.

Images adapted from [6].
Main Supervisor: Alexander Düster (TUHH)
Co-Supervisors: Björn Busse (UKE) & Imke Fiedler (UKE)
Research background and question
Besides its general impact on bone biomechanics and fracture risk, marrow fat can impact the damping properties of bones, which may contribute to bone remodeling and the development of osteoporosis [1-3]. Developing a detailed computational model based on CT scans could provide valuable insights into how marrow fat affects the mechanical properties of vertebral bone. A central method of our research is the Finite Cell Method (FCM), which is highly suitable for this project due to its ability to simulate microstructured/heterogeneous materials derived from CT scans [4,5]. This method has been effectively employed in CT-based structural analyses of bones, as demonstrated in the work by Schillinger et al. [6], see Figure above. This project aims to investigate the influence of fat in vertebral bone by employing and advancing the Finite Cell Method and Spectral Cell Method to simulate the bone’s elastodynamic behavior, elucidating a potential risk factor for fractures and metabolic bone disease.
Specific aim
- Develop a computational model based on CT scans and the FCM to study the influence of fat on the mechanical damping behavior of vertebral bone.
Work program and methods
CT scans from vertebral bones will be used as a starting point for a dynamical simulation based on the FCM. Using CT data, a spatial discretization will be derived to form the foundation of the analysis. The study will explore various modeling approaches, each with different levels of complexity, to accurately represent the presence of fat within the bone. Dynamic simulations in the time domain, which include varying volume fractions of fat, will be used to examine and understand the bone’s damping properties. This numerical model will enable us to assess whether bone remodeling can take place in order to maintain optimal damping, which may be compromised by increased fat content. Ultimately, the project will seek to determine whether such remodeling is associated with the development of osteoporosis, resulting from increased marrow fat content.
P9 Assessing musculoskeletal intervention using zebrafish locomotion analyses and tissue phenotyping
Main Supervisor: Imke Fiedler (UKE)
Co-Supervisors: Alexander Schlaefer (TUHH) & Maike Frye (UKE)
Research background and question
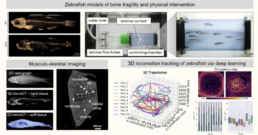
Zebrafish provide a valuable preclinical model for investigating MSK function and tissue interfaces due to large evolutionary conservation of bone, muscle, and vascular pathways [1-4]. Their small size, ease of genetic manipulation, and capacity for in vivo drug administration enable high-throughput studies of locomotion and tissue-specific phenotyping. However, comprehensive MSK phenotyping combined with deep-learning-based locomotion tracking remains a largely unexplored approach in zebrafish research [5]. Performing correlative functional analyses of the MSK system in zebrafish models of disease can help to better understand osteosarcopenic-vascular mechanisms and assess the effects of interventive measures on locomotion patterns and physical capacities. Recent work within the ICCIR has brought forward a deep-learning based imaging pipeline for efficient tracking of 3D locomotion patterns of zebrafish based on deep learning (Figure above). This zebrafish locomotion tracking pipeline has been already developed for wild type zebrafish and will be elaborated and extended in this project to correlate zebrafish MSK tissue phenotypes with locomotion patterns, musculoskeletal activity, and swimming performance.
Specific aims
- Establish zebrafish models with conditions impairing the MSK system for intelligent motion tracking and testing effects of exercise- and treatment-based intervention measures.
- Assessing bone, muscle, fat, and vascular tissue phenotypes in the models through multi-scale imaging and analytics from whole-body to ultrastructure.
- Correlate MSK tissue phenotypes of zebrafish models with locomotion patterns, musculoskeletal activity, and swimming performance.
Work program and methods
Zebrafish models of sarcopenia, bone defects and vascular disease will be used and combined to create new models of multifactorial MSK conditions. Physical exercise protocols as well as medical intervention protocols will be applied with the aim to regenerate musculoskeletal health. Disease models will be subjected deep-learning based 3D locomotion tracking to monitor physical performance and to extract disease patterns, and will undergo multi-scale ex vivo characterization of their hard and soft tissues and interfaces (e.g., bone, muscle, fat, tendons, vessels) based on multimodal imaging from the whole body (microCT) to the nanoscale (e.g., Raman imaging and spectroscopy, confocal imaging, synchrotron nanoCT, and volume electron microscopy [6], [7]).
P10 What are the sex-specific vascular-driven mechanisms contributing to changes in gait and motion?
Main Supervisor: Felix v. Brackel (UKE)
Co-Supervisors: Alexander Schlaefer (TUHH) & Maike Frye (UKE)
Research background and question
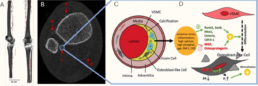
Peripheral artery disease and vascular calcification are associated with reduced tissue perfusion, muscle fatigue, altered gait patterns, and impaired movement coordination, ultimately increasing fall risk and functional decline. While bone-muscle-vascular interactions are increasingly recognized, the specific contribution of vascular calcification and remodeling to human gait and movement biomechanics remains insufficiently understood. In particular, sex-specific differences are rarely addressed in an integrated manner. Most existing studies focus on bone or muscle composition but lack functional movement assessments and detailed vascular characterization. In a previous pilot study of 300 patients at UKE, we identified sex-specific associations between lower-limb vascular calcifications and bone remodeling. However, functional neuromuscular outcomes such as gait, movement efficiency, and postural stability were not included. This project addresses this gap by combining vascular imaging, neuromuscular performance data, and computational analysis to elucidate sex-specific vascular influences on human movement.
Specific aims
- To characterize sex-specific gait and movement alterations associated with vascular calcification.
- To identify vascular and neuromuscular patterns that are associated with functional decline and increased fall risk.
- To investigate interactions between vascular health, neuromuscular performance, and bone quality using integrated imaging and functional data.
Together, these aims seek to establish vascular-driven mechanisms of altered movement patterns and to identify potential markers for early functional impairment.
Work program and methods
The project will leverage existing clinical datasets comprising HR-pQCT, DXA, and neuromuscular performance assessments. Computational workflows will be developed for automated segmentation and quantification of vascular calcifications, bone structure, and muscle-related parameters. Machine learning-based movement analysis will be applied to evaluate postural stability and dynamic gait characteristics in relation to vascular health. The integrated analysis aims to derive predictive models linking vascular and neuromuscular parameters to movement alterations and bone quality.